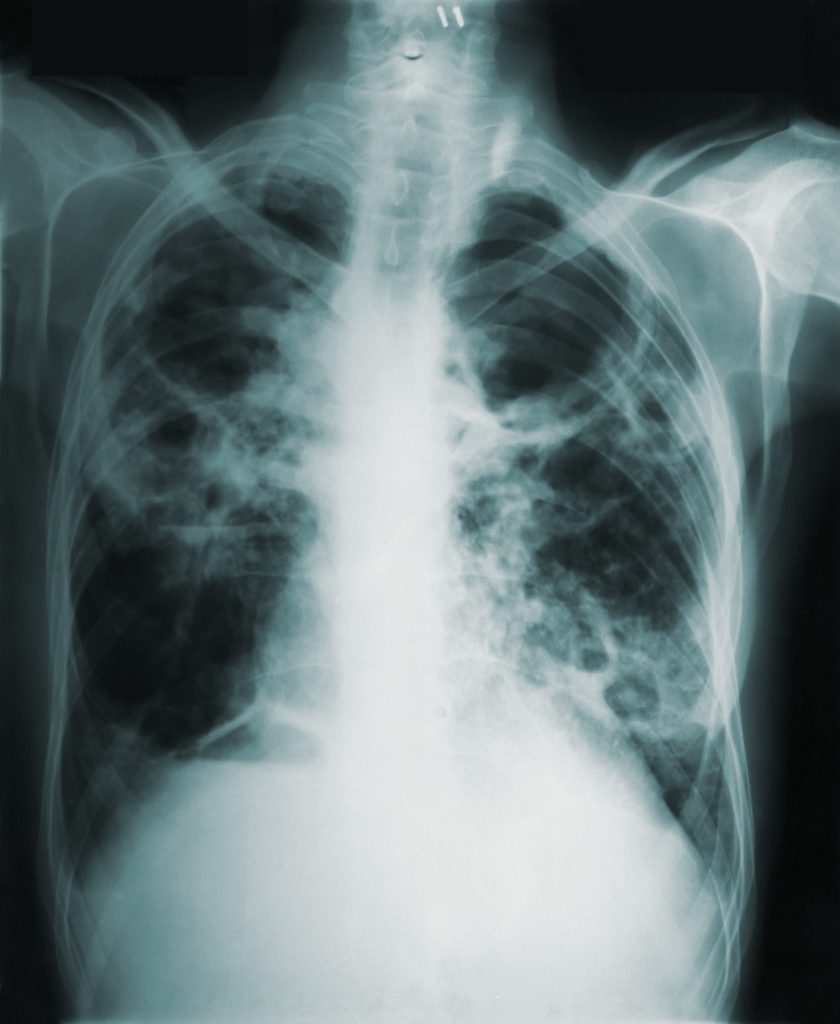
Pneumonia is a preventable and treatable lung disease that is especially deadly among the elderly, children and the immunocompromised. It is the number 1 killer of children under 5, claiming more lives in this age group than AIDS, malaria, and measles combined [1]. Despite the overwhelming death toll of pneumonia, it rarely receives coverage in the news media. This World Pneumonia Day, we are highlighting how pneumonia can be treated in low resource areas.
Pneumonia causes inflammation of air sacs within the lungs, causing respiratory distress, sickening 155 million children under 5 and killing 1.6 million each year. A chest X-ray is often used to diagnose pneumonia [2]. Blood tests, such as a complete blood count (CBC) see whether your immune system is fighting an infection. Pulse oximetry measures how much oxygen is in your blood; Pneumonia can prevent adequate oxygenation. Pulse oximeters are also used in identifying need, but health care disparities make it less suitable for darker skinned populations.
In recent years, a drastic increase has been observed in the implementation of artificial intelligence (AI) and machine learning (ML) models in medicine, especially in the field of respiratory medicine [3]. The main milestone in the implementation of AI and ML in respiratory medicine was the use of these models to predict the risk of lung cancer in 3 years by prospectively evaluating characteristics of pulmonary nodules through computed tomography (CT) scans of the chest [4]. Based on the encouraging results, the implementation of algorithms has expanded to models for the characterization of idiopathic pulmonary fibrosis with a degree of accuracy similar to radiologists with extensive expertise in high-resolution CT, as well as in the distinction between interstitial pneumonia and non-interstitial pneumonia usual [5].
Nonetheless, deep neural networks (DNNs) are a form of AI with multiple layers characterized through layers of inputs and outputs, commonly used to extract and progressively analyze a varied spectrum of information [1]. The application of DNNs within respiratory medicine has shown significant results in distinguishing pathologies such as tuberculosis, pneumonia, malignant pulmonary nodules and pneumothorax [5,6]. A study conducted by Hwang et al [7] developed a model of DNNs capable of recognizing imaging tests for lung cancer and pneumonia as well as providing the visual location of the abnormality [6]. In this same context, an algorithm created by Yates et al [8] elaborated a binary classification in “normal” and “abnormal” to analyze CT scans of the chest, as results the authors showed an accuracy in the model of 94.6%. These promising findings have important implications for the clinical management and, above all, for the quality of life of these patients as a result of the rapid institution of appropriate pharmacological or non-pharmacological treatment [9].
Despite notable advances, the good performance of AI models is largely dependent on the available and properly labeled database, as well as the diversity of associated clinical, laboratory and histopathological data, such as comorbidities, lifestyle, complementary blood tests. or biopsies, aiming to guarantee reproducible and clinically relevant results. However, despite recent challenges related to the COVID-19 pandemic, drastic advances have been observed regarding the effectiveness of AI models in distinguishing pneumonia from acute respiratory syndromes due to the increase in the number of available and varied databases on chest CT, corroborating for diagnostic efficiency and reduced radiologist workload [10].
Thus, future perspectives on the implementation of AI models unfold in the processing speed of these models and the wide range of clinical data aimed at separating pathological conditions associated or secondary to the pathology of interest.
Citations
[1] “Pneumonia.” https://www.who.int/health-topics/pneumonia (accessed Nov. 03, 2022).
[3] Kaplan A, Cao H, FitzGerald JM, Iannotti N, Yang E, Kocks JWH, Kostikas K, Price D, Reddel HK, Tsiligianni I, Vogelmeier CF, Pfister P, Mastoridis P. Artificial Intelligence/Machine Learning in Respiratory Medicine and Potential Role in Asthma and COPD Diagnosis. J Allergy Clin Immunol Pract. 2021;9(6):2255-2261.
[4] Huang P, Lin CT, Li Y, Tammemagi MC, Brock MV, Garner M, et al. Deep machine learning predicts cancer risk in follow-up lung screening. Available from: https://ssrn.com/abstract¼3384912. Accessed October 7, 2020.
[5] Walsh SLF, Calandriello L, Silva M, Sverzellati N. Deep learning for classifying fibrotic lung disease on high-resolution computed tomography: a case-cohort study. Lancet Respir Med. 2018 ;6(11):837-845.
[6] Hwang EJ, Park S, Jin K-N, Kim JI, Choi SY, Lee JH, et al. Development and validation of a deep learning-based automatic detection algorithm for active pulmonary tuberculosis on chest radiographs. Clin Infect Dis 2019;69:739-47
[7] Pasa F, Golkov V, Pfeiffer F, Cremers D, Pfeiffer D. Efficient deep network architectures for fast chest X-ray tuberculosis screening and visualization. Sci Rep 2019;9:6268.
[8] Hwang EJ, Park S, Jin K-N, Kim JI, Choi SY, Lee JH, et al. Development and validation of a deep learning-based automated detection algorithm for major thoracic diseases on chest radiographs. JAMA Netw Open 2019;2:e191095.
[9] Yates EJ, Yates LC, Harvey H. Machine learning “red dot”: open-source, cloud, deep convolutional neural networks in chest radiograph binary normality classification. Clin Radiol 2018;73:827-31.
[10] William WS. Clinical implications and challenges of artificial intelligence and deep learning. JAMA 2018;320:1107-8.